Real Life Fantasy: Hemocyanin, and the colors of blood
So we’ve been talking about the history of the color blue, and one shade in particular: murex purple. Turns out that [anthropologists and archaeologists believe] blue was the last color category to enter the human lexicon, and was likely the last color to be distinguished/perceived by human eyes.

PLUS, there’s one particular shade of blue (or indigo, depending) that is derived by extracting the blood from thousands of little ocean snails, oxidizing it, and dyeing fabric with it to create a mystical hue known as tekhelet, Tyrian purple, or (as mentioned above) murex purple, which was once more valuable than gold–partially because it became brighter when exposed to sunlight and weathering.
In the beautiful mosaic of 20th-century art and science, it was discovered how and why the blood of many earth critters can manifest so many beautiful hues.
Hemoglobin is what we humans (and most mammals) have as a means to carry oxygen to the cells in our bodies. It uses iron molecules to get the job done.
Hemocyanin, on the other hand, uses copper to do this same job in many sea creatures, including crabs, lobsters, and of course, sea snails.
Wait, copper? Like, the stuff pennies were made of?

Yep.
So how do we get blue dye from copper? I bet you’re asking.
Oxygen, and sunlight. Really! When copper oxidizes*, it turns a greenish-bluish shade.
What do you mean, you don’t believe me? You’ve seen the Statue of Liberty, right?

That French beaut is made of 3/32 in copper, protected by a lovely patina. Totally rockin’ that look, Lady Liberty!
But here’s the part that was news to me: some animals have green blood! Others have purple blood! This Vox article explains this phenomenon well.
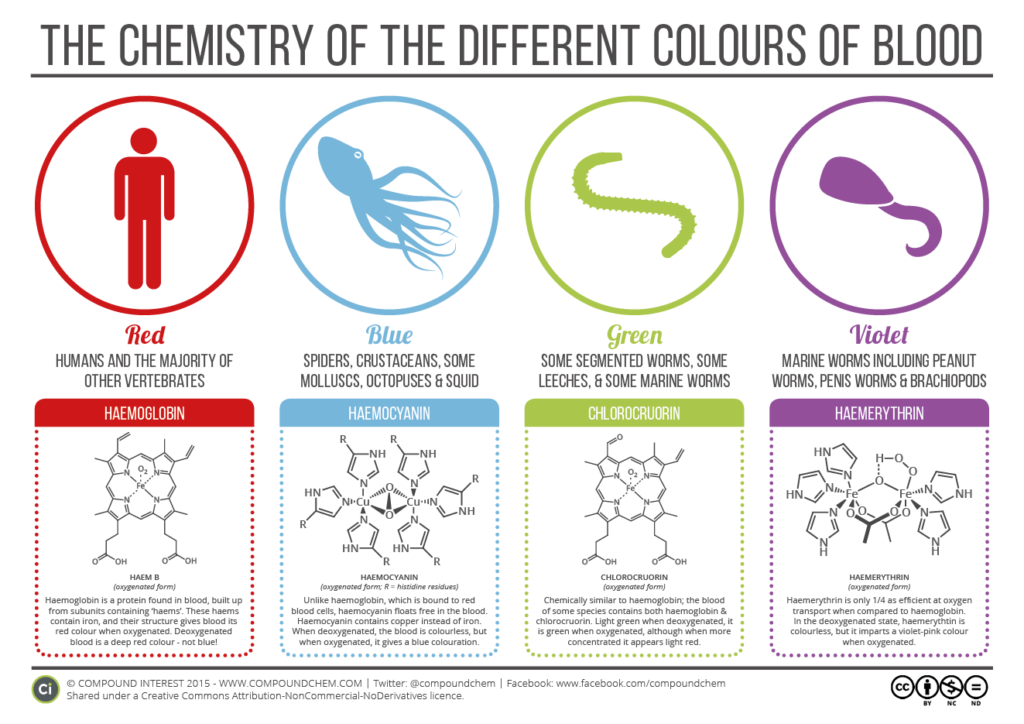
There’s no mention of animals being able to draft, though. I thought for sure there would be some mention that dissection revealed these creatures were packing luxin… Huh, I just realized sub-red drafters give whole new meaning to “packing heat”!
Okay, I’m gonna stop there.
*Thanks for making us do all those redox equations in AP Chem, Ms. Johnson! That knowledge finally came in handy! 😉
